There are three separate methods for obtaining an FDA Prior Notice Certification number:
1) Utilize the FDA Website at https://www.access.fda.gov.
It is possible (but highly unlikely) that your importers will obtain the FDA PNC and provide it to you along with full and complete entry documentation, reforwarding instructions, and any collect freight charges and duties due. If ABI is down, you MUST submit PN data via the FDA website. Instructions are available on the site. If you do happen to receive the PNC via this method, there is a field on the FDAED5 screen to enter this data.
2) Utilize the WP application through ABI.
This is an alternate method that AIRPEX users can utilize. This application allows you to obtain a PNC number as a separate function from the entry process. Use it to get PNC numbers for I.T.s, outport shipments where you want to provide PNC data, or on shipments that you cannot transmit to ABI because you are missing I.T. numbers or similar data. You must still provide complete FDA line item details with this method.
3) Submit full details on each FDA line item on the entry via ABI.
----------------------------------------------------------------------------------------------------
Basic steps to obtain an FDA PNC number in AIRPEX:
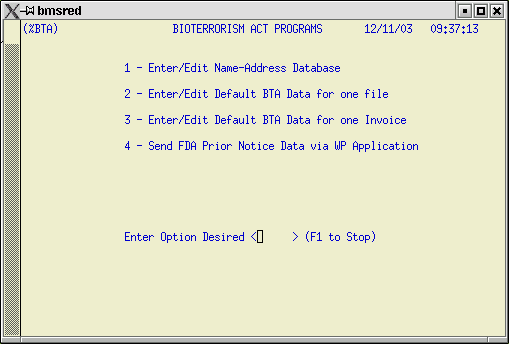
1) Go to the new %BTA menu.
2) Populate the BTA Name/Address Database.
3) Prepare the entry as usual. HTS numbers flagged with FD3 or FD4 require FDA Prior Notice Certification. Enter the BTA Name/Address database key in the new BTA MFG and BTA SHPR fields on the FDA Edit screen.
4) Upload the entry. A new question during the validation process will ask if you want to also upload BTA data. Answer 'Y', add any required data and complete the upload.
5) The entry is accepted/rejected by ABI.
-----------------------------------------------------------------------------------------------------
New/Changed screens:
1) BTA Menu - %BTA
2) BTA Name/Address Database Listing - %BTADBIQ
3) BTA Name/Address Database Details - %BTADBED
4) BTA Default Data for 1 File - %BTAFLDF
5) BTA Default Data Override for 1 Invoice - %BTFLINV
6) FDA Data Entry Screen - %FDAED5
7) FDA Default Data Override Single Line Item - %BTFLLN
8) Customer Part Number Data/FDA Data Entry Screen - %FDAED5
-------------------------------------------------------------------------------------------------------
1) %BTA
The new BTA Menu screen can be accessed by typing BTA into the Select Option field from the ABI Operations menu. Use this screen to select options to create or edit the BTA Name/Address database, create BTA data for entries/invoices, and transmit PNC data to ABI via the WP application.
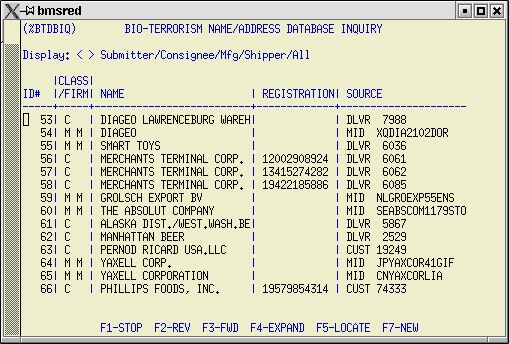
2) BTA Name/Address Database Listing - %BTADBIQ
Prior Notice Certification requires that the details in this file (name, address, contact, email, etc.) for the submitter of the data, the consignee, the shipper, and manufacturer be submitted for each FDA line item with a FD4 designation. Use this screen to create and maintain the BTA Name/Address file. It works the same as other database listings in AIRPEX. Type in your search criteria, then use F5 to locate. Use F4 to expand to the detail screen. Use F7 to add a new record.
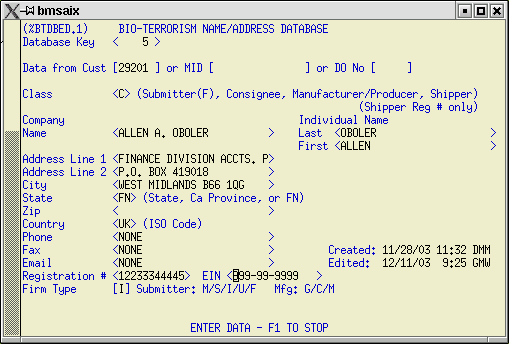
3) BTA Name/Address Database Details - %BTADBED.
Use this screen to enter details about the submitter, consignee, manufacturer, and shipper. The first entry in this database should be your company, since it will always be considered the 'Submitter' for FDA PNC purposes. You can extract this information from the Customer, MID, or Delivery Customer databases. Place the cursor in the appropriate field on the Data from: line and look up information from there. Unfortunately, the record structure required by Customs for this information does not exactly match the record structure required by FDA, so some manipulation of the data will probably be required. Make sure that the Class and Firm type are filled in for all classes of entities. Registration number is required for manufacturers and shippers. Both Registration number and EIN (IRS) number are required for importers and consignees. First and last name, phone, fax, and email address of contact are required. Blanks in these fields will cause entries to be rejected. Use 'UNKNOWN' for first and last name, if this is the case. Use 'NONE' for the email field. Do not use special character such as dashes ( - ) or parentheses ( ) in phone or fax number fields.
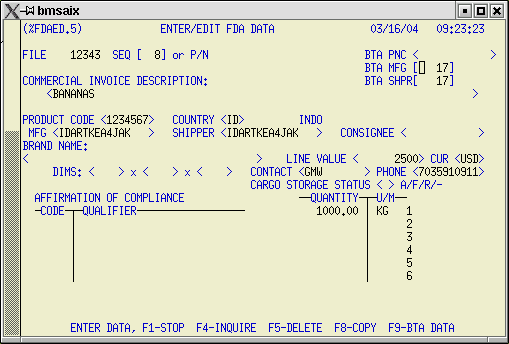
4) FDA Edit Screen - %FDAED.5
We have added three new fields to the FDA Edit screen: BTA PNC, BTA MFG, and BTA SHPR.
If you have already obtained a PNC number from your importer, from another broker, or through the WP Application, just add the PNC number in the BTA PNC field. No other data entry is required.
To obtain the PNC number in conjunction with submission of your Customs entry, fill in the BTA MFG and BTA SHPR fields. These are linked to the BTA Name/Address database which can be accessed using the F4 key.
If the shipper and manufacturer are the same for all FDA line items on the entry, it is not necessary to fill in these fields on subsequent FDA line items. In fact, if the shipper and manufacturer are the same for all FDA line items on the entry, you don't even need to complete these fields at all. You can designate default data for the entire entry on the BTA Default Data screen during the upload procedure. The system will take this default information and apply it to each FDA line item on the entry.
When you have multiple manufacturers or shippers on your entry the system considers the first ones entered to be the default for the entire shipment. What this means is that you only need to enter BTA MFG/BTA SHPR data on line items that are different from the default. Blank line items will have the default data applied to them during the upload process.
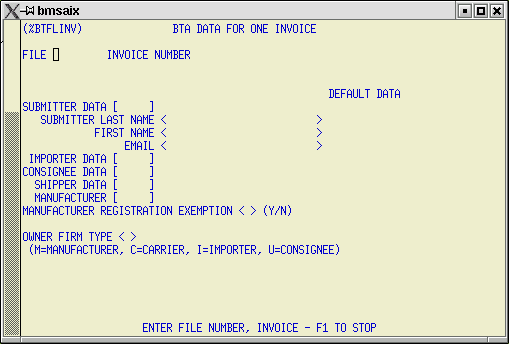
5) BTA Default Data for a single invoice.
If your entry has multiple invoices with different BTA MFG/BTA SHPR data and each invoice has multiple FDA line items from that manufacturer/shipper combination, you can enter default BTA data for complete invoices using this screen.
Default data for the entire file is displayed on the right hand side of the screen. Default data for the exception invoice is entered in the fields on the left.
The data entered here will be applied to each FDA line item associated with this one single invoice. File level default data will be applied to all FDA line items on the entry except those linked to the invoice specified on this screen.
You can use this screen to name exceptions to the default BTA data for as many invoices as required.
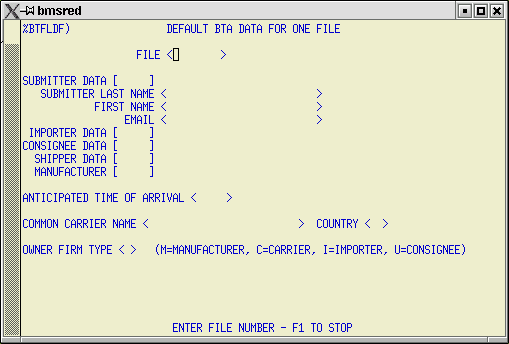
6) BTA Default Data - %BTAFLDF.
During the upload process on entries that contain FDA3 or FDA4 flagged items you will be asked if BTA data should be appended to the file for transmission to Customs. When you answer 'Y' this screen is displayed. Use this screen to enter/confirm default BTA data for the file.
Confirm or enter Submitter, Importer, Consignee, Shipper, and Manufacturer data and fill in any other required fields. Arrival time is required for all entry and MOT types. If shipment is arriving by rail, the border crossing point and railcar/container number fields must be filled in. Continue with the upload process using the Shift F4 keystroke combination.
The system will apply this data to each FDA line item on the entry unless exceptions have been created at the invoice or line item level. When the entry passes all other validations it will be queued up for the next transmission to ABI.
ABI edits the entry and BTA data and sends acceptance or rejection messages. These messages are displayed in the Transmission Results screens. If all the BTA data submitted is in order, the entry is processed as usual. An FDA prior notice certification number will be assigned for each FDA line item. These show up on the Transmission Results screen labelled as BN records. The PNC numbers are then automagically inserted into the appropriate fields in the entry.